
Technical Facilities
The laboratory's research relies on a range of techniques and spectroscopies to synthesize, characterize and test the systems developed by our researchers. These state-of-the-art facilities belong either to the laboratory or to associated organizations, such as the Fédération de Chimie et Matériaux de Paris-Centre (FCMat).
Please find below a presentation of these, as well as the LRS contacts:
Optical, vibrational and Electronic Spectroscopies Facilities
Contacts : Jean-Marc KRAFFT and Laetitia VALENTIN
Principle - Benefits of the technique
Infrared is a spectroscopic method for analyzing vibration modes, providing information on the molecular structure of materials. By analyzing the modes of vibration of adsorbed probe molecules, this technique can also be used to characterize certain surface properties of materials (acidity, basicity, metallic character, etc.).
The spectroscopic equipment consists of 7 BRUKER SPECTROMETERS (2 Vector 22, 2 Vertex 70, 1 Vertex 80, 1 Tensor II and 1 Invenio) for routine analyses and others more specific to the laboratory's themes.
In situ analysis in transmission mode:
Two spectrometers (1 Vector 22 and 1 Vertex 70) dedicated to in situ analyses enable the monitoring of heat treatments and the characterization of material surface properties (acidity, basicity, coordination mode, metal dispersion, nature of active sites, ...) via the adsorption-desorption of probe molecules (CO, NO, Pyridine, Propyne, Acetylene, N2, labeled molecules, ...).
Spectrometers :
- Vector 22: mid-IR range 370-7500 cm-1; DTGS detector optimum resolution: 1 cm-1
- Vertex 70: mid-IR range 350-8000 cm-1; DTGS and MCT detectors optimum resolution: 0.4 cm-1
Accessories & environment:
- Each spectrometer is equipped with specific cells developed for measurements in transmission mode under controlled atmospheres at RT (Figure a) or at liquid nitrogen temperature (Figure b) after in situ heat treatment of the material (under vacuum or controlled atmosphere).
- Samples in the form of self-supporting pellets are placed on a mobile sample holder, inserted into the IR cell. This sample holder allows the operator to move the sample either in the oven, for thermal treatment, or in the IR beam, for analysis.
- Each measuring cell is connected to a glass manifold (c), enabling its atmosphere to be controlled (vacuum or gas flow treatment, introduction of probe molecules).
- Selected publications
- FTIR with probe molecules: Pyridine, CO (low T) & UV spectr.
Sadek, R.;Chalupka-Spiewak, K.; Krafft, J.-M.;Millot, Y.; Valentin, L.; Casale, S.;Gurgul, J.; Dzwigaj, S.
The Synthesis of Different Series of Cobalt BEA Zeolite Catalysts by Post-Synthesis Methods and Their Characterization. Catalysts, 12, 1644, 2022; https://doi.org/10.3390/catal12121644
- FTIR with proble molecules: Lutidine, CO (Low T) & Raman spectr. & acidity-catalytic activity correlation
Lebarbier V., Houalla M., Onfroy T.
New insight into the development of Brönsted acidity of niobic acid. Catal. Today, 192, 123-129, 2012; https://doi.org/10.1016/j.cattod.2012.02.061
- FTIR with proble molecules: Pyridine
Armenise S., Costa C., Luing W. S., Ribeiro M. R., Silva J. M., Valentin L., Casale S., Onfroy T., Muñoz M. and Launay F.
Design and evaluation of two approaches for the synthesis of hierarchical micro-/mesoporous catalysts for HDPE Hydrocracking. Microporous Mesoporous Mater., 353, 112605, 2023; https://doi.org/10.1016/j.micromeso.2023.112605
- FTIR with proble molecules N2 (Low T) & Raman spectr.
Jeffrey T. Miller, Neil M. Schweitzer, Mimoun Aouine, Philippe Vernoux, Abdelmalik Boufar, Juliette Blanchard, Jean-Marc Krafft, Christophe Méthivier, Céline Sayag, Frédéric Ser, Mickaël Sicard, and Cyril Thomas
Successive Strong Electrostatic Adsorptions of [RhCl6]3− on Tungstated-Ceria as an Original Approach to Preserve Rh Clusters From Sintering Under High-Temperature Reduction. J. Phys. Chem. C, 125, 25094−25111, 2021; https://doi.org/10.1021/acs.jpcc.1c07644
- FTIR with proble molecules: CO (Low T) & NMR
Gairola P., Millot Y., Krafft JM., Averseng F., Launay F., Massiani P., Jolivalt C., Reboul J.
On the importance of combining bulk- and surface-active sites to maximize the catalytic activity of metal–organic frameworks for the oxidative dehydrogenation of alcohols using alkyl hydroperoxides as hydride acceptors. Catalysis Science & Technology; 10, 20, 6935-6947, 2020; https://doi.org/10.1039/D0CY00901F
- FTIR isotopic exchange; Probe molecules: CO (Low T)
S. Diallo-Garcia, M. Ben Osman, J-M. Krafft, S. Boujday, G. Costentin.
Discrimination of infra red fingerprints of bulk and surface POH and OH of hydroxyapatites. Catalysis Today, 226, 81 - 88, 2014; http://dx.doi.org/10.1016/j.cattod.2013.11.041
In situ/operando analysis:
Two spectrometers are mainly dedicated to the analysis of powders under pre-treatment (in situ) or reaction (operando) conditions:
- Vertex 70: IR range 350-8000 cm-1 or 130-6000 cm-1 (KBr or wide range beamsplitters); DTGS and MCT detectors; optimum resolution: 0.16 cm-1 ; 56 scans/sec at 16 cm-1 or 42 scans/sec at 8 cm-1
- Tensor II: mid-IR range 200-5500 cm-1; MCT detectors; optimum resolution: < 2 cm-1
Dedicated accessories :
- DRIFT cells with reaction chamber
- HTC (High temperature cell) transmission cell
The Bruker Tensor II spectrometer is mobile, enabling it to be coupled with catalytic tests for in situ/operando measurement campaigns. For mechanistic studies, the use of labeled reagents can also be envisaged.
- Selected publications
- DRIFT In Situ
L. Lin, D. Cornu, M.M. daou, C. Domingos, V. Herledan, J-M. Krafft, G. Laugel, Y. Millot, H. Lauron-Pernot
Role of Water on the activity of Magnesium Silicate for Transesterification Reactions. ChemCatChem, Volume 9, Issue 12, 2399-2407, 2017; https://doi.org/10.1002/cctc.201700139
- DRIFT Operando
Ben Osman, M; Krafft, JM; Thomas, C; Yoshioka, T; Kubo, J; Costentin, G.
Importance of the Nature of the Active Acid/Base Pairs of Hydroxyapatite Involved in the Catalytic Transformation of Ethanol to n-Butanol Revealed by Operando DRIFTS. ChemCatChem, Volume11, Issue 6, Pages 1765-1778, 2019; https://doi.org/10.1002/cctc.201801880
Time-resolved measurements at solid-gas and solid-liquid interfaces
Contact: Alberto MEZZETTI
A Bruker Vertex 80 spectrometer is dedicated to time-resolved measurements in transmission or attenuated total reflectance (ATR) mode.
- Vertex 80: IR range 350-8000 cm-1; DTGS and MCT detectors; optimum resolution < 0.2 cm-1; rapid scan: acquisition speed > 110 scans/sec at 16 cm-1
Dedicated accessories :
- Diamond crystal ATR (ATR platinium)
Routine analyses :
- Invenio: mid-IR range 350-8000 cm-1 ; DTGS detectors; optimum resolution: < 0.5 cm-1
Contact : Jean-Marc KRAFFT
Principle - Benefits of the technique
Raman spectroscopy is a scattering spectroscopy. Like IR spectroscopy, it probes the vibrational states of the compound under analysis at the molecular and/or crystalline level. But as the selection rules are different from those of IR spectroscopy, these two spectroscopies are in fact complementary.
Equipment :
Kaiser Optical System, Raman Analyzer RXN1 microprobe equipped with a 785nm laser diode.
Features:
Laser diode: l = 785 nm; Max power: 400 mW
Range 100 to 3450 cm-1 on one acquisition
Resolution 4 cm-1 / 3 pixels
1024x256-pixel CCD detector, Peltier-cooled (-70°C)
Two possible modes:
1) Microraman by coupling the spectrometer to a LEICA microscope
Objective 10X; 50X long working distance; 100X
2) Macro using a remote measuring head (5m fiber optics) which can be equipped with a very long focal length lens (75 mm) or a 10X lens.
In macro mode, the spectrometer is mobile. In this configuration, it can be used for In Situ/Operando measurements on laboratory set-ups (catalytic tests, synthesis stations, etc.) or for coupling with other available characterization techniques (FTIR, Photoluminescence, etc.).
- Selected publications
- Raman In Situ & EPR
Sarah Petit, Thomas, Yannick Millot, Frederic Averseng, Dalil Brouri, Jean-Marc Krafft, Stanislaw Dzwigaj, Gwenaelle Rousse, Christel Laberty-Robert and Guylène Costentin.
Synergistic Effect Between Ca4V4O14 and Vanadium-Substituted Hydroxyapatite in the Oxidative Dehydrogenation of Propane. ChemCatChem,13, 3995–400, 2021; https://doi.org/10.1002/cctc.202100807
- SERS (Surface Enhanced Raman Spectroscopy)
Vincent Pellas, Juliette Blanchard, Clément Guibert, Jean-Marc Krafft, Antoine Miche, Michèle Salmain and Souhir Boujday.
Gold Nanorod Coating with Silica Shells Having Controlled Thickness and Oriented Porosity: Tailoring the Shells for Biosensing. ACS Appl. Nano Mater, 4, 9, 9842–9854, 2021; https://doi.org/10.1021/acsanm.1c02297
- Raman & fluorescence spectr.
Palierse E., Hélary C., Krafft JM., Génois I., Masse S., Laurent G.,Alvarez Echazu M.I., Selmane M., Casale S., Valentin L., Miche A., Chan B.C.L., Lau C.B.S., Ip M., Desimone M. F., Coradin T., Jolivalt C.
Baicalein-modified hydroxyapatite nanoparticles and coatingswith antibacterial and antioxidant properties. Materials Science & Engineering C; 118, 111537, 2021; https://doi.org/10.1016/j.msec.2020.111537
- Raman & FTIR probe molecules: N2 (Low T)
Jeffrey T. Miller, Neil M. Schweitzer, Mimoun Aouine, Philippe Vernoux, Abdelmalik Boufar, Juliette Blanchard, Jean-Marc Krafft, Christophe Méthivier, Céline Sayag, Frédéric Ser, Mickaël Sicard, and Cyril Thomas.
Successive Strong Electrostatic Adsorptions of [RhCl6]3− on Tungstated-Ceria as an Original Approach to Preserve Rh Clusters From Sintering Under High-Temperature Reduction. J. Phys. Chem. C, 125, 25094−25111, 2021 ; https://doi.org/10.1021/acs.jpcc.1c07644
Contacts : Josefine SCHNEE and Frédéric AVERSENG
Principle - Benefits of the technique
This spectroscopy records electronic absorption spectra of compounds in solution (transmission) or in solid form (reflection), and identifies the electronic configuration of the element in question, if it's a metal, as well as its environment (number and nature of ligands, symmetry of the complex), by means of the number of bands and their position.
The near-infrared section provides complementary information to that gathered by infrared spectroscopy, in terms of bond vibrations (harmonic bands of IR bands, or combinations). Using the mantis equipment, it is also possible to work in reflection on a solid placed in an environmental cell, subjected to heat treatments and/or changes in gaseous atmosphere.
Equipment:
Cary 5000 (Varian) equipped with a 150 mm external sphere for acquisition of diffuse reflection spectra on powders, over a 190-2500 nm range.
In addition to conventional equipment for working in transmission or with a mantis cell/environmental cell device (temperature, gas atmosphere or vacuum treatments).
Exemple:
As you can see in the picture below, the shift in bands towards longer wavelengths is due to the weaker ligand field created by the oxide ions on an alumina surface, compared with that created by the nitrogen atoms in ethylenediamine.
Above: Evolution of the [Ni(en)3]2+ complex deposited on alumina and heated under helium; evidence of progressive grafting of the complex to [Ni(en)2(OAl)2].
Contact : Frédéric AVERSENG
Principle - Benefits of this technique
Electron paramagnetic resonance (EPR or Electron Spin Resonance ESR) is a spectroscopy very similar in concept to NMR. It can be used to identify and study paramagnetic species (with unpaired electrons) present in liquid, solid or gaseous media. It is typically used to detect and/or characterize organic radicals, transition metal complexes/ions, point defects in solids or conduction electrons. The very high sensitivity of this technique is in the ppm range.
In the case of transition ions/complexes supported on oxides, the spectra obtained can be used to determine the degree of oxidation, the number of ligands, the symmetry, the position (surface or core) and the dispersion of these paramagnetic species, providing a better understanding of the activity of heterogeneous catalysts.
In addition to these qualitative characterizations, EPR can also be used quantitatively in liquids (e.g. production of hydroxyl radicals by Fenton reaction) or pseudo-quantitatively for solids (e.g. creation/disappearance of defects during chemical/thermal treatments).
In addition, temporal EPR monitoring can be used to characterize the appearance/disappearance kinetics of paramagnetic species (e.g. species stability, photochemical reactions, ...).
Equipment : JEOL FA-300 (2008) operating in X-band and equipped with a magnet capable to reach up to 16,000 Gauss (1.6 T).
Contact : Jean-Marc KRAFFT
Principle - Benefits of the technique
Photoluminescence spectroscopy probes the electronic states of a material by analyzing the photon emission (near UV, visible, near infrared) of the photoexcited material, as well as by analyzing the photo excitation (often UV, visible).
Photoluminescence can occur in all forms of matter (gas, solid, liquid).
There are two types of luminescence following photon excitation (photoluminescence): fluorescence, which is generally characterized by a light emission following excitation after a very short time (≈ 10-8s), and phosphorescence, which corresponds to an emission offset from excitation by a longer time (10-5s to several minutes).
Equipment :
Horiba Jobin Yvon Fluorolog® -3. FL3-22
Features:
Xe 450W lamp and pulsed lamp; excitation spectrometer (double monochromator); emission spectrometer (double monochromator); detection (RT) photomultiplier (R928P).
This equipment can be used to perform :
- emission spectra: measurement of emission over a given spectral range for a fixed excitation energy.
- excitation spectra: measurement of emission at a given energy as a function of excitation energy over a spectral range.
- emission decay versus time measurements for given emission and excitation energies.
Dedicated environment and accessories :
- equipment for measurements on solutions (tanks) and solids
- controlled atmosphere measurements on solids (dynamic vacuum, etc.)
- low-temperature (77 K: liquid N2) or very low-temperature measurements on solids (T ≈ 20K)
- Selected publications
- Controlled Formation of Native Defects in Ultrapure ZnO for the Assignment of Green Emissions to Oxygen Vacancies
Zhang M; Averseng, F; Krafft, JM; Borghetti, P; Costentin, G; Stankic, S
The Journal of Physical Chemistry C, 124, 12696−12704, 2020
https://dx.doi.org/10.1021/acs.jpcc.0c01078
- Baicalein-modified hydroxyapatite nanoparticles and coatingswith antibacterial and antioxidant properties
Palierse E., Hélary C., Krafft JM., Génois I., Masse S., Laurent G.,Alvarez Echazu M.I., Selmane M., Casale S., Valentin L., Miche A., Chan B.C.L., Lau C.B.S., Ip M., Desimone M. F., Coradin T., Jolivalt C.
Materials Science & Engineering C; 118, 111537, 2021
https://doi.org/10.1016/j.msec.2020.111537
- Identification and Distribution of Surface Ions in Low Coordination of CaO Powders with Photoluminescence Spectroscopy
H.Petitjean, J-M. Krafft, M. Che, H. Lauron-Pernot, G. Costentin
Journal of Physical Chemistry C, 115, 3, 751-756, 2011
https://doi-org.inc.bib.cnrs.fr/10.1021/jp110193k
Surface Analysis Facilities
Contact : Christophe MÉTHIVIER
Principle - Benefits of the technique
Infrared is a spectroscopic method for analyzing vibrational modes, providing information on the molecular structure of materials. By analyzing the vibrational modes of probe molecules, this technique can also be used to characterize certain surface properties of materials (acidity, metallic character, etc.).
- 3 Nicolet spectrometers are dedicated to reflectance mode analysis (ATR, IRRAS, PM-IRRAS).
Contacts : Christophe METHIVIER and Antoine MICHE
Principle - Benefits of this technique
The principle of XPS ( X-ray photoelectron spectroscopy) is to analyze the kinetic energy of electrons produced by the ionization of elements in a solid irradiated with a monochromatic X-ray beam. By measuring the kinetic energy (Ec) of the photoelectrons and knowing the irradiation energy (hv), the binding energy of the electrons (El) can be directly determined by the simple relation of the energy conservation.
This technique therefore gives direct access to the electronic structure of the various elements constitutive of the material. The low mean free paths of photoelectrons in solids make XPS a surface analysis technique. Typically, in the kinetic energy range used (below 1400 eV), the information obtained comes from a material thickness of much less than 10 nm. This technique therefore mainly provides information on the relative proportions of the elements present in the first atomic layers and on their oxidation state.
At the LRS, XPS coupled with other surface techniques such as Low Energy Electron Diffraction (LEED), Auger Electron Spectroscopy (AES), and Infra-Red Reflection Absorption Spectroscopy) (IRRAS) is used for surface science studies. It is also widely used to characterize self-assembled layers, biological interfaces and catalytic materials.
Two XPS spectrometers are routinely used at the LRS:
- The first, coupled with other surface characterization techniques (LEED, AES, PM-IRRAS), is equipped with several devices for depositing inorganic or organic materials (E-Beam evaporator, Knudsen cell, electrospray source), enabling the study of deposit-substrate interfaces under quasi in situ conditions.
- The second, owned by the FCMat Federation, is semi-automated and more dedicated to "routine" analyses for the characterization of catalytic materials, hybrid materials, polymer films, self-assembled layers, bio-interfaces, battery materials, semiconductors... This device is equipped with an enclosure that enables heat treatments to be carried out in a controlled atmosphere.
Contact : Antoine MICHE
Principle - Benefits of this technique
This technique is mainly used to measure the wettability of a liquid relative to a surface. In particular, it enables to check the success of grafting on surfaces, non-destructively and at low cost.
The analysis method involves placing a drop of solvent on a surface and measuring the contact angle between the surface and the wall of the drop of solvent formed.
Fig. 1 : Contact angle (q) and interfacial tensions (g) of a drop deposited on a solid surface.
A direct result will give the hydrophilic or hydrophobic character of the surface by comparing the angles obtained. Let's take the example of a drop of water deposited on a COOH- or COO-terminated thiol layer. The COOH-terminated layer has a higher contact angle than the COO-terminated layer (55° vs. 48°), indicating its more hydrophobic character.
This process also enables the surface energy of a solid to be measured. Surface energy or interfacial tension (g) is calculated from a number of contact angle measurements between different liquids and the solid to be analyzed. Knowing the surface tensions of the liquids, it is thus possible to trace the surface energy of the solid and verify, for example, the success of a chemical functionalization of the studied surface.
Fig. 2 : 1 µL drop of water deposited on a COOH-terminated thiol surface in (a), q = 55° ; and on a COO-terminated thiol surface in (b) q =48°.
Équipement : DSA 100 Easy Drop, Krüss GmBH
Contact : Souhir BOUJDAY
Principle - Benefits of this technique
Microscopy Facilities
Contact : Jean-Marc KRAFFT
Principle - Benefits of this technique
Contact : Jessem LANDOULSI
Principle - Benefits of this technique
Atomic Force Microscopy (AFM) is used to study surface topography with down to atomic-scale resolution.
The basic element of this technique is a flexible tip with a radius of curvature of a few nanometers. This tip is in contact with the sample, which is itself attached to a piezoelectric ceramic scanner:
When a voltage is applied to these ceramics, they expand or contract, enabling the sample to be moved in all three directions of x,y,z space to an accuracy of the order of 0.1 nm. A laser is reflected off the AFM tip and collected in a four-dial photodiode. This measures the deflection (dials a and b) and torsion of the AFM tip (c and d). When the flexible tip encounters an obstacle, it can bend and the laser changes position in the photodiode. A feedback loop also controls the force with which the tip is pressed against the sample. The position of the laser in the dials thus gives direct access to the topography of the sample.
There are 3 AFM operating modes:
- Contact mode
- Non-contact mode, in which the tip oscillates on the surface of the sample without ever coming into contact with it
- Tapping (or intermittent contact) mode, in which the tip oscillates intermittently in contact with the sample.
In the contact mode used in this work, the tip and sample are in permanent contact. Thanks to the feedback loop, the force normal to the surface can be kept constant at a minimum value (<500 pN).
Equipment : Bruker
AFM applications in biology :
AFM is a technique that has revolutionized the study of surface structure and properties. It is the only method that gives direct access to the height of structures (z direction). Furtherly, unlike other high-resolution methods (e.g. transmission electron microscopy), its use requires no invasive sample preparation.
A fundamental aspect of AFM is its ability to work in liquid, often aqueous, media. This advantage makes AFM the method of choice for characterizing biological objects in native, physiological conditions, in real time and with unrivalled resolution. In fact, this technique has made it possible to provide 3-D images of biological structures, including biomolecules, lipid films, proteins, nucleic acids, whole cells, and so on. Force spectroscopy also provides access to physical surface properties such as elasticity, hydrophobicity and intra- and inter-molecular interactions.
Force spectroscopy has been applied to the study of numerous interactions, for example, in the study of specific interactions between avidin and biotin or for the mapping of adhesion on the surface of living cells by grafting a specific ligand onto the AFM tip.
Analytical Facilities
Contact : Céline SAYAG
Principle - Benefits of this technique
Mass spectrometry is a physical analysis technique used to detect and identify gaseous molecules by measuring their mass.
Its principle is to produce ions in the gas phase using an ionization source, and then to separate these charged gaseous molecular ions according to their mass-to-charge ratio (m/z). This ionic current is converted into an electric current, the signal of which is processed by computer to obtain data in the form of a mass spectrum: Relative abundance = f(m/z).
Two compact Pfeiffer OmniStar GSD 320 mass spectrometers (below) are available in the laboratory. They can detect masses from 1 to 100 amu (m/z) using Faraday or SEM detectors.
Being "mobile", i.e. on a rolling table, they can be coupled to all kinds of experimental devices such as: TGA-TDA, TPR, TPO, DRIFT, catalytic tests... using a flexible capillary with Swagelok connectors that can be heated to a maximum of 200°C.
This makes it possible to monitor the chemical species formed during isothermal or temperature-programmed pre-treatments under neutral, reducing or oxidizing gases and atmospheric pressure.
Each mass spectrometer has its own computer, and mass spectra are recorded (=f(tps, T°C, m/z) and processed using Pfeiffer's QUADERA software.
The techniques described below can be used to study the chemical and physical behavior of solid samples during heat treatment.
TGA and DSC thermal analysis
Contacts : Xavier CARRIER and Dalila SEGHOUANE
Principle - Benefits of this technique
Simultaneous TGA - DSC analysis measures both mass variations and heat fluxes in a sample as a function of temperature or time in a controlled atmosphere.
Equipment: SDT Q600 from TA Instruments
- Thermogravimetric Analysis TGA :
Thermogravimetric Analysis (TGA) is a thermal analysis technique that measures the quantity and rate of mass change of a sample as a function of temperature and time in a controlled atmosphere.
Example: TGA study of calcium oxalate monohydrate
Exp. conditions : Ca(C2O4).H2O, 17,6070 mg ; 25°C to 1000°C at 20°C/min ; 100mL/min N2 flow
- Differential Scanning Calorimetry (DSC):
Differential Scanning Calorimetry (DSC) measures temperatures and heat flows associated with thermal events in a material as a function of time and temperature in a controlled atmosphere. These measurements provide qualitative and quantitative information on physical and chemical transformations involving endothermic or exothermic heat exchanges.
Note: DSC is identical to DTA in principle, but works, as indicated, on energy differences rather than temperature differences.
Example: Coupled TGA-DSC study of calcium oxalate monohydrate
Exp. conditions : Ca(C2O4).H2O, 17,6070 mg ; 25°C to 1000°C at 20°C/min ; solid line (--) under air, dotted line (- -) under N2. Heat flows are shown in blue.
Laboratory use and analysis:
These two analysis techniques are often used to determine the characteristics of samples, such as: degradation temperatures, hydration rates, quantities of organic and inorganic compounds and, above all, identification of the temperature ranges in which thermal events occur. Performing this type of analysis in air also enables us to observe sample oxidation.
Mass spectrometry coupling (TGA/DSC - MS):
At LRS, we have chosen to couple our SDT Q600 instrument to a mass spectrometer, which enables us to complete the TGA and DSC analyses of the sample by identifying, at the thermobalance outlet, the products or gases released by the transformation during the thermal cycle.
Equipment: Pfeiffer Thermostar GDS 301T3
Example: MS-coupled TGA study of calcium oxalate monohydrate
Exp. conditions : Ca(C2O4).H2O, 17,6070 mg ; 25°C to 1000°C at 20°C/min
TPO/R/D
See also in Chemisorption/physisorption section
Temperature Programmed Reduction (TPR)
The material is heated under a flow of a reducing H2/Ar mixture. Measuring hydrogen consumption with temperature gives quantitative information on the reducibility of the species present.
Temperature Programmed Oxidation (TPO)
The material is heated under a flow of an O2/He oxidizing mixture. Measurement of oxygen consumption with temperature provides quantitative information on the oxidizability of the species present.
Temperature Programmed Desorption (TPD)
Contact: Juliette BLANCHARD
The material is exposed to a gas stream containing species that can adsorb specifically to surface sites (e.g. NH3 on acidic sites). Tracking desorption with temperature provides information on the quantity of sites present and the strength of their interaction with the adsorbed species.
Equipment : Micromeritics Autochem
Synthesis Technical Assistance
Contact : Guylène COSTENTIN
Principle - Benefits of this technique/device
The laboratory is equipped with a METTLER TOLEDO Optimax 1001 automated synthesis reactor (500 or 1000 mL), which can be used to synthesize solids by precipitation. This device is controlled by a software that enables a series of sequential actions to be carried out, with adjustment of various parameters (T, pH, stirring speed, volumetric additions of liquid reagents) and recording of all reaction parameters throughout the experiment.
Liquid reagents, acids or bases are added via 2 volumetric pumps.
Reactor temperature is programmable and controlled by Pelletier effect (rapid response to exothermic events). Mechanical stirring is programmable. The pH reading is corrected for the temperature in the reactor, and its value can be adjusted during the reaction.
The reaction can be carried out under inert gas flow, and reflux can be adapted. Reaction monitoring by in situ Raman spectroscopy (remote probe) is possible thanks to the transparency of the reactor window to the laser beam.
At the end of the experiment, a valve at the base of the reactor allows easy recovery of the solution.
Adorption, Desorption and Catalytic Properties Characterization Facilities
Contact : Souhir BOUJDAY
Principle - Benefits of this technique
The resonant frequency of a crystal depends on the amount of material deposited on its surface. The principle of the quartz crystal microbalance (QCM) is based on measuring the frequency of a quartz crystal on whose surface a thin, rigid layer of material is deposited. The variation in frequency is directly proportional to the variation in mass due to the molecules adsorbed on the surface. It is also proportional to the density and viscosity of the liquid in contact with the quartz.
This technique makes it possible to determine in situ, whether in the gas or liquid phase, the quantity of material adsorbed on the crystal surface, with a detection limit of 1 ng/cm2 for 5 MHz quartz in the gas atmosphere, and 5 ng/cm2 in the liquid. As macromolecules (proteins, polymers, etc.) deposited on a surface do not form a thin, rigid layer, their viscoelastic behavior induces a loss of vibration energy through dissipation. By acquiring data at different harmonics, we can determine the viscoelastic characteristics of the deposited layers, rule out frequency variations due to dissipation of vibration energy, and calculate the mass deposited on the quartz surface from a model.
Equipment: Q-SENSE E1
Dissipation measurements (QCM-D)
This device can be used to study molecular interactions and adsorption on all types of surfaces (gold, TiO2, SiO2, etc.). A special chamber enables liquid-phase measurements under flow and controlled temperature. Applications include measurements of the growth of protein layers, polyelectrolytes, cell-bacteria interactions or any type of target on molecular receptors (biosensors).
Contacts : Souhir BOUJDAY and Antoine MICHE
Principle - Benefits of this technique
Surface plasmon resonance imaging (SPRi) is an optical sensing technique used to monitor and analyze biomolecular interactions in real time. The technology measures changes in refractive index at the surface of the chip, which can be correlated to changes in mass. It can be used to detect interacting molecules in real time, to determine the concentration of the analyte and the affinity of the interaction. Binding, or mass accumulation, induces a change in refractive index and a shift in the position of the resonance angle. SPRi tracks reflectivity variations occurring at a fixed angle (working angle) as a function of time.
The imaging capability enables users to visualize the entire working area, and to work in a format that allows different types of ligand to be immobilized on the same surface. It also enables many parameters to be studied at the same time (concentration, immobilization pH, etc.), making it easy to compare, rank and select molecules, for work in the field of bio-interfaces (biosensors).
- Step A: Ligands are immobilized as a network on the functionalized chip surface.
- Step B: When the sample solution is injected into the cell, molecular binding can occur. This induces a shift in the plasmonic curves and an increase in reflectivity. Kinetic curves show variations in reflectivity as a function of time. The process can also be followed on the SPRi difference image. The white dots correspond to the interaction zones of the chip.
- Step C: When the sample solution leaves the cell, the ligand-analyte complexes dissociate. This induces a shift in the plasmonic curves and a decrease in reflectivity. Kinetic curves show variations in reflectivity as a function of time. The process can also be followed on the SPRi difference image, with the interaction spots becoming darker.
- Step D: When all ligand-analyte complexes have been completely dissociated (using a regeneration solution), the plasmonic and kinetic curves return to the initial state. The SPRi difference image is black again.
Equipment : HORIBA Scientific-GenOptics SPRi-PLEX II system
Physisorption and chemisorption are two types of adsorption that play a key role in the characterization of solids: physisorption, which is based on a weak, non-specific interaction between the molecule and the surface, is used to characterize the entire surface of a solid; chemisorption, which is stronger and more specific, is used to characterize specific sites on the surface.
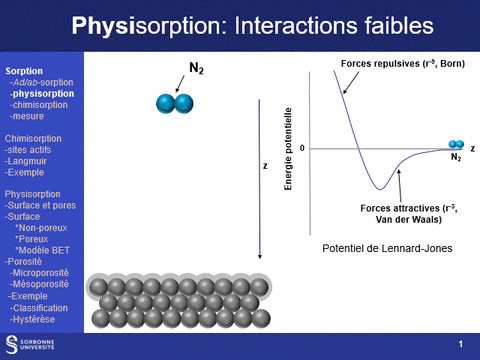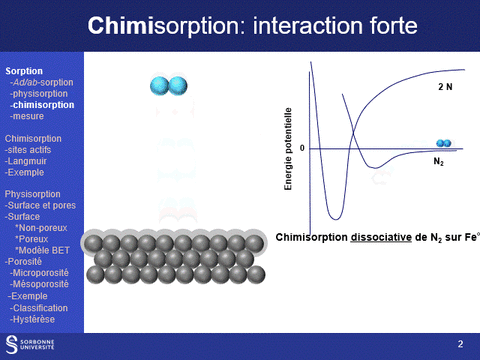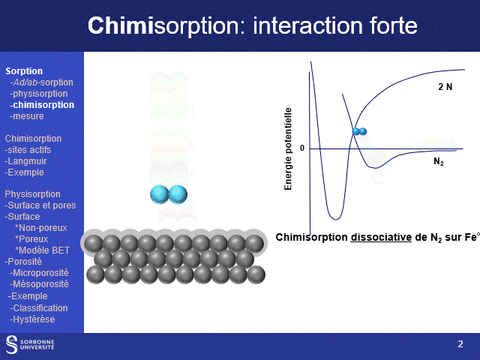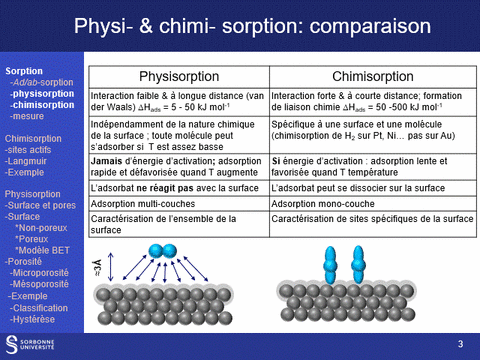
Equipment available at LRS: (a) ASAP 2020 (physi- (N2 and CO2)- and volumetric chemisorption; (b) BelSorp Max physisorption of N2 and Kr; (c) physi- (N2) and volumetric chemisorption (H2, CO, O2) ; (d) TPR, TPO, TPD (CO2, NH3) and pulsed chemisorption (H2, CO, O2); (e) physisorption (N2 and Kr @N2 liq, Ar & Kr @Ar liq), hysteresis loop scanning
Chemisorption
Principle - Benefits of this technique
Gas or vapor chemisorption is widely used in heterogeneous catalysis to quantify and evaluate the strength of active sites (acid, metal, etc.) on catalyst surfaces. This can be done dynamically (pulsed chemisorption) or by measuring adsorption isotherms (volumetric or gravimetric measurement). The probes routinely available at LRS are H2, CO, O2 (to measure metal sites) and NH3 and CO2 (to measure acid and base sites respectively).
It is also possible to characterize surface sites using temperature programmed experiments:
- Temperature Programmed desorption (TPD): the material is exposed to a gas stream containing species that can adsorb specifically to surface sites (typically NH3 on acidic sites or CO2 on basic sites). Tracking desorption with temperature provides information on the number of sites present and the strength of their interaction with the adsorbed species.
- Temperature Programmed Reduction (TPR): the material is heated under flow of a reducing H2/Ar mixture. The measurement of hydrogen consumption with temperature provides information on the quantity of reducible species and their reducibility.
- Temperature Oxidation Programmed (PTO): the material is heated under a flow of an O2/He oxidizing mixture. Measurement of oxygen consumption with temperature provides information on the quantity of oxidizable species and their oxidizability.
One of the devices can also be used to measure breakthrough curves (on small sample volumes). These experiments characterize the adsorption capacity of a material for a given molecule, and involve exposing the material to a flow of that molecule and monitoring its detection at the reactor outlet.
Equipment :
- Volumetric chemisorption : BelsorpMax from Bel Japan
Contacts : Cyril THOMAS and Juliette BLANCHARD
- Pulsed chemisorption, TPR, TPO, TPD, breakthrough curves : Autochem III, Micromeritics associated to an OmniStar mass spectrometer
Contact : Saremblé GUIRA
Physisorption
Principle - Benefits of this technique
Gas physisorption (N2, Ar, Kr, CO2) can be used not only to determine the specific surface area of a solid, but also to analyze its porosity, by determining microporous (pores with diameters of less than 2 nm, particularly in the case of zeolite family materials) and mesoporous (pores with diameters between 2 and 50 nm) volumes, as well as pore diameter distributions in these two domains. It can also be used to determine the accessibility of this porosity.
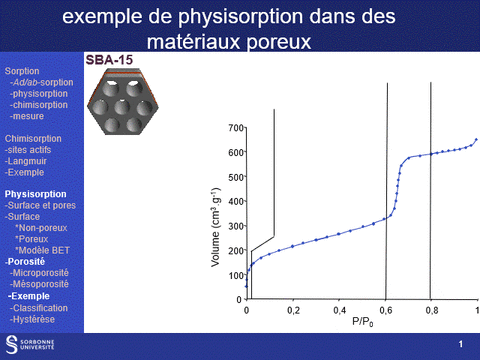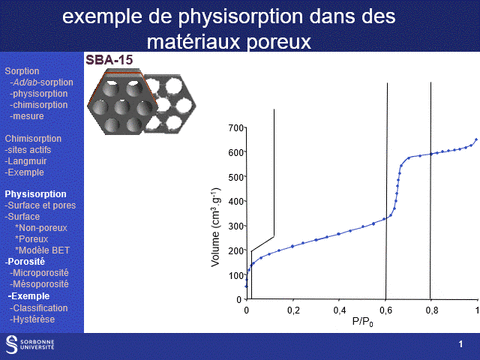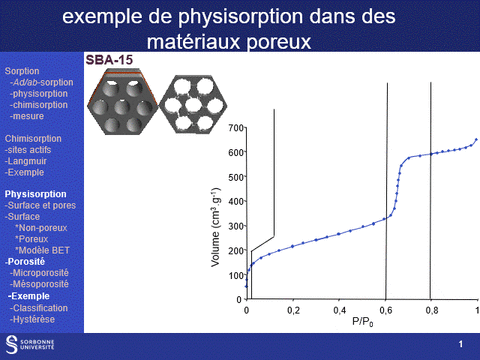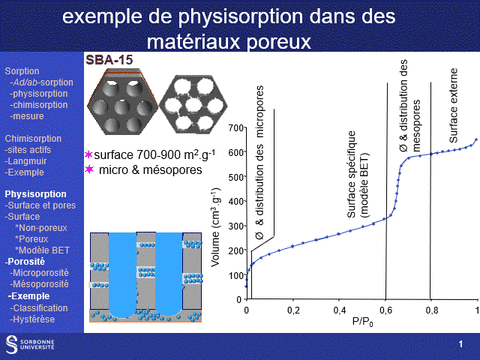
These properties are fundamental characteristics of materials, particularly for applications in the field of heterogeneous catalysis, since the physical properties that govern the use of a material as an active phase support are (i) its specific surface area: a high surface area favors a high dispersion of the supported phase and (ii) its porosity, which governs the diffusion of reactants and products within the catalyst grains and which can also, when the pore diameter is close to the size of the reactants, modify the selectivity of reactions.
A physisorption instrument measures the volume of an adsorbed gas (usually nitrogen) at a constant temperature (e.g. liquid nitrogen, 77K) as a function of the gas pressure. To achieve this, the sample must first be degassed by a vacuum pre-treatment adapted to its chemical nature.
The adsorption isotherm thus obtained is characteristic of the sample's porosity. It can be used to characterize the microporosity and mesoporosity of the materials studied: specific surface area, pore distribution, pore volume. Analysis of the hysteresis loop provides information on the accessibility of this porosity (particularly from hysteresis loop scanning experiments).
Equipment:
The three devices dedicated to physisorption are suitable for microporosity and mesoporosity measurements with N2 as adsorbate at the temperature of liquid N2. In addition,
- the single-user ASAP 2020 (Micromeritics) is used for CO2 adsorption at different temperatures.
Contact : Franck LAUNAY
- the 2 (or 3) station BelsorpMax, Bel Japan is used for Kr adsorption at liquid N2 temperature. It is therefore suitable for measuring samples with low specific surface area.
Contact : Saremblé GUIRA
- the 3 Flex 3-station instrument (Micromeritics, DIM RESPORE funding) is equipped with a cryostat (CryoTune, 3P) for measuring isotherms between 82 and 135 K. This device is particularly well suited to measuring Ar and Kr adsorption isotherms at liquid Ar temperature (Kr isotherms at liquid Ar temperature are used to determine the porosity of samples with low specific surface area). For this type of measurement, only 1 analysis port is used. In addition, this device can be used in "hysteresis loop scanning" mode for advanced porosity studies.
Contacts : Julien REBOUL and Juliette BLANCHARD
Contact : XX
Principle - Benefits of this technique
Electrochemistry Facilities (In Progress)
Principe - Intérêt de la technique
Contact : Vincent VIVIER
FCMat Federation Platform
Contacts : Yannick MILLOT and Virginie HERLEDAN
Principle - Benefits of this technique
NMR is a spectroscopic technique based on the study of the behavior of atoms' nuclear spins when placed in an intense magnetic field and subjected to a radiofrequency field.
The various interactions present (chemical shift, scalar and dipolar coupling, quadrupole interactions and their anisotropic aspects in the solid state) provide a wealth of information, particularly on the structure of materials.
The atoms studied may be part of the catalyst or adsorbed molecules.
In the case of catalytic materials of interest to the laboratory, the vast majority of studies concern aluminum-27 (nuclear spin value I = 5/2) and silicon-29 (I = 1/2), but a great deal of work is being carried out on other nuclei (1H, 13C, 51V...).
Equipment : The LRS has access to the NMR spectrometers of the Fédération de Chimie et Matériaux de Paris-Centre (FCMAT). This includes two Bruker ultrashield 700 SB and 500 WB spectrometers for solid-phase NMR, and a Bruker 500 SB spectrometer for liquid-phase NMR.
Contact : Juliette BLANCHARD
Principle - Benefits of this technique
X-ray powder diffraction is used to identify crystalline phases and to assess sample crystallinity and crystallite size.
Although the laboratory does not have its own X-ray diffractometer, it has access to those of FCMAT.
Contacts : Dalil BROURI and Sandra CASALE
Principle - Benefits of this technique
In the field of catalysis, and more generally of materials, transmission electron microscopy is a technique that provides morphological (micrographs) and structural (electron diffraction) information on solids from the nanometer to the tenth of a nanometer scale. It can be used to analyze the shape and size of crystallites and surfaces, highlight inter-growths or identify the distribution of phases within a solid.
In the case of porous materials (zeolites, mesoporous materials, etc.), it can be used to visualize the regularity of channel systems, check the location of any particles dispersed in the porosity and measure their size histograms. The microtomy technique, which enables thin sections to be taken, is also very useful for analyzing the very core of crystallites and checking their homogeneity.
Equipment : LRS members have access to FCMAT microscopes.
